Acid rain is any form of atmospherically deposited acidic substance containing strong mineral acids of anthropogenic origin. It was reportedly first described in England by Robert Angus Smith in 1872. Acid rain is more properly called acidic deposition, which occurs in both wet and dry forms. Wet deposition usually exists in the form of rain, snow, or sleet but also may occur as fog, dew, or cloud water condensed on plants or the earth's surface. Dry deposition includes solid particles (aerosols) that fall to the earth's surface. Condensation of fog, dew, or cloud water is referred to as occult deposition.
The most common acidic substances are compounds containing hydrogen (H + ), sulfates (SO = 4 ), and nitrates (NO 3 ). The chief source of these compounds is the combustion of fossil fuels such as coal, petroleum, and petroleum by-products, primarily gasoline. Agriculture is also a major source of nitrates. Power plants that burn coal contribute over 50 percent of sulfates to the atmosphere and 25 percent of nitrates.
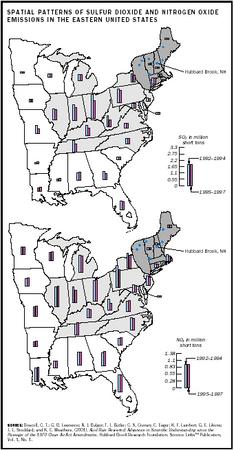
Prior to the Clean Air Act of 1970, acid deposition was mostly a local problem confined to the immediate vicinity of the pollution source. After 1970, emitters of acidifying pollutants increased the height of smokestacks to reduce local pollution by diluting pollutants in larger volumes of air. The result was the regional transport of acid deposition to remote locations. Acid rain has adversely affected large areas of the mountainous regions of the eastern United States and Canada, Scandinavia, central and Eastern Europe, and parts of China. Areas that are downwind of heavy concentrations of power plants receive the most deposition.
Acid rain acidifies soils with low calcium carbonate levels, which results in the acidification of water passing through the soil to streams and lakes. Calcium carbonate soil-buffering capacity is related to soil origin. Soils weathered from rocks high in calcium carbonate have high calcium carbonate buffer capacity. Fish and other aquatic life have been eliminated from streams and lakes by acid deposition. Continued acid deposition leaches calcium and magnesium from the soil and results in the increased mobility of aluminum, which is toxic to both animals and plants. Aluminum is always present in soils, but it is innocuous until mobilized into soil water by acidic deposition. Its presence in water in small amounts will cause the outright

Acid forest soils are thought to cause forests to decline and grow more slowly. Soil acidity causes nutrient deficiencies in trees and other plants and predisposes them to attack by pathogens such as insects and fungi. Soil acidity also increases photo-oxidant stress in plants. Monuments and buildings made of marble or other forms of calcium carbonate and statuary made of certain metals such as copper are also damaged by acid deposition. The acidification of waters leads to increases in mercury uptake by fish, causing them to be unsafe to eat.
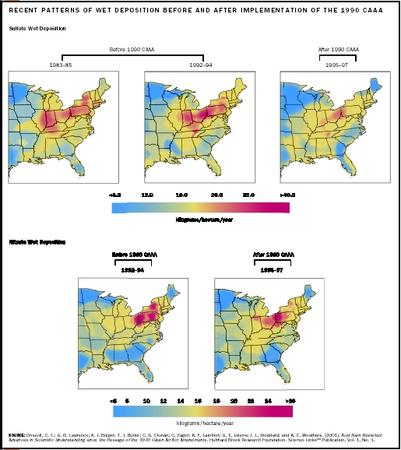
The governments of the European Economic Community, Canada, and the United States have taken steps to reduce the emissions of sulfate and nitrates. The Clean Air Act Amendments of 1990 were designed to reduce U.S. emissions of sulfate by about 40 percent through a program of emissions trading between emissions generators, use of low-sulfur coals (fuel switching), and controls on power plant smokestack emissions. Although this program has significantly reduced acidic deposition in many parts of the northeastern United States, many scientists agree that additional reductions will be required to prevent continued damage and allow for meaningful recovery of affected lakes and streams.
SEE ALSO A IR P OLLUTION ; C OAL ;
Internet Resource
Environment Canada Web site. Available from http://www.ec.gc.ca/acidrain .
William E. Sharpe






0 comments:
Post a Comment