© 2011 Henry Auer
Global warming is the rise in the average temperature of Earth's atmosphere and oceans since the late 19th century and its projected continuation. Since the ...
Thursday, 28 April 2011
Esty and Porter Recommend Imposing a Price on Carbon Emission in the U. S.
© 2011 Henry Auer
Tuesday, 19 April 2011
Carbon Dioxide – The Waste Product of Our Energy Economy
© 2011 Henry Auer
Thursday, 14 April 2011
Producing More Natural Gas in the U. S.: The Pickens Plan
Natural gas is the most efficient of the fossil fuels, emitting the least CO2 for the amount of heat energy obtained. It can be used in electricity plants as well as to fuel cars and trucks when the engine is properly modified. Nevertheless, natural gas fuel still emits CO2. Optimally we should move to renewable energy as soon as possible.
© 2011 Henry Auer
Diesel
Rudolf Christian Karl Diesel (1858–1913), a German thermal engineer, invented the diesel engine and patented it in 1893. Unlike their gasoline counterparts, which ignite an air/fuel mixture using spark plugs, diesel engines compress air to a very high pressure and then inject the fuel. The fuel then ignites due to the high temperature of the compressed air. Diesel engines are relatively fuel-efficient engines commonly used in heavy construction equipment, ships, locomotives, commercial trucks, and some large pickups, as well as in the production of electricity at some power plants or in factories.
Diesel-powered automobiles gained popularity in the United States during the oil crisis of the 1970s because they tend to result in better fuel economy than their gasoline counterparts. But diesel-powered cars have declined in popularity with American drivers since their peak in the mid-1970s because of quality-related problems in early models and because earlier diesel engines did not accelerate as quickly as those powered by gasoline. Diesel passenger cars have also declined in popularity because they are more expensive and they emit more smog-forming pollutants and toxic soot than other conventional internal-combustion engines. For eighteen-wheel trucks and other large vehicles, however, diesel engines are currently the standard.
Consumer Pollution
Consumer pollution refers, in part, to traces of numerous consumer products, including pain relievers, prescription drugs, antibiotics, insect repellent, sunscreens, and fragrances—collectively called pharmaceuticals and personal care products (PPCPs)—discovered in inland and ocean waters. Between 1999 and 2000 the United States Geological Survey (USGS) established the widespread occurrence in the environment of minute but measurable quantities of PPCPs, along with other organic wastewater contaminants, such as detergent metabolites , plasticizers, and fire retardants. These contaminants were discovered in 80 percent of 139 waterways downstream from sewage treatment plants and livestock operations. Before 1999 most research into PPCPs took place in Europe, and pharmaceuticals were detected there in sewage treatment effluent, surface water, groundwater, drinking water, and the North Sea.
How PPCPs Enter the Environment
Thousands of PPCPs are consumed worldwide, some on a par with agrochemicals, at rates of thousands of tons a year. These substances enter the environment largely from sewage treatment plants, as well as directly from fish farms, storm runoff, recreational activities, and leaking landfills. Incompletely metabolized drugs or their metabolites—chemicals formed from the body's interaction with the drug—are excreted in the urine and feces of humans and animals. Cosmetics and perfumes are washed off in the shower. Unused drugs are flushed down the toilet or thrown out in the trash. Antibiotics, steroids, and other drugs that are used to treat animals, such as those in confined animal feeding operations, are eventually washed into sewer drains or directly into local waters from roads and farms.
Sewage is treated to break down human waste, but the wide occurrence of eighty-two out of the ninety-five substances tested for in the USGS survey indicates that many synthetic chemicals are not completely removed by sewage treatment methods. PPCPs in wastewater effluent end up in rivers, lakes, and oceans, and contaminants in manure or sewage sludge are spread on land.
Potential Health and Environmental Effects
The concentrations of PPCPs found in the USGS survey range from parts per trillion (ppt) to parts per billion (ppb), or nanograms per liter to micrograms per liter. For pharmaceuticals this is many orders of magnitude below the concentrations prescribed as medication.
However, some PPCPs, such as musk fragrances, are fat soluble and so can bioaccumulate in animal tissue. In addition, many water-soluble PPCPs are effectively persistent if they are continually replenished from sewage effluent. Fish and other aquatic species could therefore be permanently exposed to these chemicals. Although research on the environmental effect of PPCPs is sparse, some studies link low concentrations of oral contraceptives, along with reproductive estrogens, to the feminization of male fish. When exposed to the estrogens, male fish produce vitellogenin, an egg protein, usually found only in the females. Other studies show that fluoxetine, marketed as Prozac, affects reproductive hormonal activity in zebra mussels, crayfish, and fiddler crabs. Some scientists think that aquatic species could be harmed

| Percentage of streams in which contaminant was found | Category of contaminant | Representative substances found (median concentration, in ppb) |
| SOURCE: Frequency of Detection of PPCPs in Streams. (2002). "Pharmaceuticals, Hormones and Other Organic Wastewater Contaminants in U.S. Streams. 1999–2000: A National Reconnaissance." Environmental Science and Technology 36, no. 6:202–1211 | ||
| 89% | Steroids | Cholesterol (0.83), coprostanol (fecal steroid) (0.88) |
| 81% | Nonprescription drugs | Acetaminophen (0.11), caffeine (0.081), ibuprofen (0.2), cotinine (nicotine metabolite) (0.05) |
| 74% | Insect repellent | DEET (0.06) |
| 66% | Disinfectants | Phenol (0.04), triclosan (0.14) |
| 48% | Antibiotics | Erythromycin metabolite (0.1), ciproflaxin (0.02), sulfamethoxazole (0.15) |
| 37% | Reproductive hormones | 17-alpha-ethynyl estradiol (0.073) (birth control), estrone (0.027) |
| 32% | Other prescription drugs | Codeine (0.012), dehydronifedipine (antianginal) (0.012), diltiazem (0.021) (antihypertensive), fluoxetine (0.012) (antidepressant) |
| 27% | Fragrances | Acetophenone (0.15) |
more by pollutants if they are exposed to efflux pump inhibitors (EPIs). EPIs decrease the cell's ability to expel potentially harmful substances and are prescribed to help drugs pass through cell walls.
Another concern is that fish and other species constantly exposed to mixtures of low levels of PPCPs will be affected in small, undetectable ways that lead to irreversible changes over time. Such small shifts in behavior, immunology, and reproduction may be attributed to natural adaptation and may not be recognized as a consequence of pollution. Seventy-five percent of streams in the USGS survey contained more than one contaminant, and 13 percent contained more than twenty.
The presence of antibiotics in 48 percent of the streams and potentially in soil that is overlaid with sewage sludge could be a factor in the increase in antibiotic resistant strains of bacteria.
There is also a potential threat to human health. Recharging groundwater from surface water constantly infused with treated sewage effluent could result in PPCP contamination in drinking water. Some pharmaceuticals, including clofibric acid, have been detected in parts per trillion in drinking water in Germany. Clofibric acid is a metabolite of drugs taken by many people to reduce cholesterol levels in the blood. The long-term effect of swallowing very low, subtherapeutic amounts of multiple medications simultaneously many times a day for a lifetime is unknown.
Current and Proposed Research
Scientists in Germany are researching treatment methods to remove PPCPs from wastewater before it enters rivers and oceans, and from potable water. Results to date show that ozone treatment and/or filtering water through granular activated carbon effectively removes the pharmaceuticals commonly found in drinking water in Berlin.
Research in the United States in 2002 is aimed at establishing where and in what concentration organic wastewater contaminants, including PPCPs, are found. PPCPs are not currently part of any water-monitoring program in the United States. The USGS is conducting a survey of these contaminants in groundwater and in sources of drinking water. Other research is focused on the presence of pharmaceuticals, including anticancer drugs and anticonvulsants in wastewater and drinking water.
Some scientists are proposing research to find out which PPCPs are harmful, especially to aquatic species, and how these chemicals work at the molecular level. Scientists also want to study whether exposure to a combination of different pharmaceuticals at low concentrations, especially those that work in the same way, poses a risk to humans or wildlife. Research is needed to develop analytical methods sensitive enough to detect traces of all the different pharmaceuticals and active ingredients in personal care products and to develop new ways for measuring subtle rather than acute toxic environmental changes.
Prevention and Recycling / Reuse
Source reduction, recycling, and replacement with "green" pharmaceuticals could help reduce excess or unused, expired medications. Disposal advice on packaging could recommend that drugs be recycled or disposed of in a controlled manner. Reducing the number of pills per container could reduce the amount of expired medication. Drug manufacturers could also find ways to minimize excess expired inventory.
Regulations
In the United States, the Food and Drug Administration requires an environmental assessment for new drugs when manufacturers predict that one ppb or more will enter the environment. The drug is then tested for acute toxicity, such as whether it causes cancer. Although most individual contaminants measured in the USGS study had concentrations below one ppb, the maximum total concentration of the thirty-three suspected or known hormonally active compounds was fifty-seven ppb. Some scientists argue that individual concentrations may not be significant for predicting risk, but concentrations of all drugs that behave in the same way should be considered.
Health Canada is at the beginning of a regulatory process that will require environmental assessment of products regulated under Canada's Food and Drug Act.
In Europe, since 1998, pharmaceuticals used in veterinary medicine must be tested for their environmental effect before they can be registered, unless the concentration predicted to enter groundwater is less than 0.1 ppb or less than ten ppb for drugs in soil. Similar standards are being considered for regulating pharmaceuticals taken by humans. Testing includes checking for inhibited algal growth, for acute, chronic, or bioaccumulation exposure in fish, for reproductive changes in birds, for earthworm toxicity, and for plant growth.
Burn Barrels
People used to think that burning household trash and yard waste in an open barrel was an inexpensive, good way to get rid of it. However, today's packaging and products are often made from plastics, dyes, and other synthetics. When burned, these cause air pollution and, in a number of U.S. states and municipalities, it is illegal. Burn barrels operate at relatively low temperatures, typically at 400 to 500° Fahrenheit (F) and have poor combustion efficiency (municipal incinerators run in the 1200 to 2000° F range).
As a result, many pollutants are generated and emitted directly into the air. Backyard trash and leaf burning often release high levels of toxic compounds, some of which are carcinogenic . Smoke from burning garbage often contains acid gases, heavy metal vapors, carbon monoxide and other sorts of dangerous toxins. One of the most harmful pollutants released during open trash burning is dioxin, a known carcinogen associated with birth defects. Dioxin can be inhaled directly or deposited on soil, water, and crops, where it becomes part of the food chain. Research has demonstrated that a single burn barrel can generate as much dioxin as a municipal incinerator serving thousands of households.
Carbon Monoxide
Carbon monoxide is an invisible, odorless, and poisonous gas with the chemical formula CO. Because of its toxicity, the U.S. Environmental Protection Agency (EPA) regulates CO. The gas is a by-product of incomplete combustion (burning with insufficient oxygen). Its major source is vehicle exhaust (60 percent). Other sources include water heaters and furnaces, gas-powered
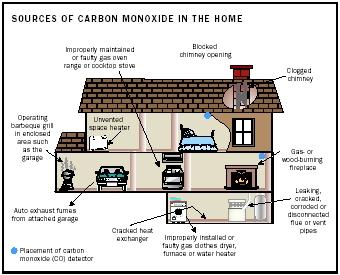
CO is classified as an indirect greenhouse gas. It does not contribute to global warming directly, but leads to the formation of ozone. Ozone is the major air pollutant formed in photochemical smog and a potent greenhouse gas.
Human exposure to elevated CO impairs oxygen uptake in the bloodstream. Under CO-free conditions, oxygen is transported from the lungs to tissues by hemoglobin. When CO is present, it mimics the shape of oxygen and binds instead to the hemoglobin. The molecule is not easily released, blocking further oxygen uptake, and ultimately depriving organs and tissues of life-sustaining oxygen. The symptoms of CO poisoning range from dizziness, mild headaches, and nausea at lower levels to severe headaches, seizures, and death at higher levels.
The EPA national outdoor air quality standard for CO is nine parts per million or ppm (0.0009 percent) averaged over an eight-hour period. The gas is life-threatening after three hours at 400 ppm (0.04 percent) and within minutes at 1.28 percent. In 1996, 525 deaths in the United States were attributed to unintentional and 1,988 deaths to intentional CO poisoning.
Exposure to CO can be reduced by assuring adequate ventilation when near any combustion source. Indoor cooking with charcoal and running gaspowered engines inside a garage are both dangerous and should be avoided. Fuel-burning appliances and fireplaces ought to be routinely inspected.
CO detectors are available to detect less obvious sources, such as a malfunctioning furnace. The sensors operate in one of three ways: They mimic the body's response to CO (biomimetic detectors), they allow a heated metal oxide to react with the gas (metal oxide detectors), or they facilitate a reaction using platinum electrodes immersed in an electrolyte solution (electrochemical detectors). The lowest level that a CO alarm can detect is 70 ppm.
Cleanup
The cleanup of environmental pollution involves a variety of techniques, ranging from simple biological processes to advanced engineering technologies. Cleanup activities may address a wide range of contaminants, from common industrial chemicals such as petroleum products and solvents , agricultural chemicals and metals, to radionuclides . Cleanup technologies may be specific to the contaminant (or contaminant class) and to the site. This entry addresses the cleanup of contaminated soil and water. Air pollution is addressed generally at the point of release by control technologies, because the opportunities to capture and recover airborne contaminants are limited once they are released into the atmosphere.
Cleanup costs can vary dramatically depending on the contaminants, the media affected, and the size of the contaminated area. Much of the

Many industry-specific cleanup programs (e.g., Florida's dry cleaner program) are funded by taxes or fees levied on that industry. Several Western European countries have environmental programs that are at least as aggressive as those in the United States. Countries with emerging economies are working hard to address environmental contamination with limited resources. Many cases of environmental contamination in former Warsaw Pact , for example, are associated with former Soviet military bases. In Poland, cleanup of several of these bases is under way. In Kluczewo, northwestern Poland, a former military base is reportedly the biggest and most contaminated such site in Central Europe. A skimming technique was used to remove liquid petroleum fuel from the subsurface followed by bioremediation of the remaining contaminated soil. The Polish government paid for the work with support from local sources.
Government involvement in environmental remediation includes consideration of the safety of the cleanup workers. Professionals involved in the cleanup of contaminated sites may have long-term exposure to a variety of hazardous materials and, as such, must be protected against adverse health

In most cases, it is financially or physically impractical to completely remove all traces of contamination. In such cases, it is necessary to set an acceptable level of residual contamination. Evaluating experimental toxicity data and then extrapolating to potential exposure scenarios forms the basis for such decisions. The result of these evaluations is an estimate of risk for given adverse outcome (e.g., cancer or death). Risk-based target levels typically determine when cleanup is complete. As a result, evolution of cleanup technologies has yielded four general categories of remediation approaches: (1) physical removal (with or without treatment); (2) in situ conversion by physical or chemical means to less toxic or less mobile forms; (3) containment ; and (4) passive cleanup, or natural attenuation . Combinations of technologies may be used at some sites.
Physical Removal
The physical removal of contaminated soil and groundwater has been, and continues to be, a common cleanup practice. However, physical removal does not eliminate the contamination, but rather transfers it to another location. In ideal cases, the other location will be a facility that is specially designed to contain the contamination for a sufficient period of time. In this way, proper removal reduces risk by reducing or removing the potential for exposure to the contamination. Removal options vary dramatically for soil and groundwater, as described below.
Soils. Excavation of contaminated soils works well for limited areas of contamination that are close to the ground surface. Under ideal conditions, the disposal location is a designed, regulated, and controlled disposal facility (e.g., a landfill or incineration facility). Alternatively, contaminated soil may be excavated and consolidated in a prepared facility on-site. Prepared disposal facilities range from simple excavations with impermeable covers (caps) to sophisticated containment structures such as those used in modern landfills. Landfills typically consist of multiple layers of impermeable materials—often combinations of synthetic (plastic) liners and compacted layers of dense clays; piping to collect and transport liquids generated within the landfill (leachate); and systems of sensors within and surrounding the landfill to detect leaks. When contaminated soil is excavated , transported, and disposed of properly, physical removal can be an effective and economical cleanup option.
Treatment of excavated soil, to either destroy the contaminant or to reduce its toxicity or mobility often is associated with physical removal. Treatment following removal will differ with the chemical of concern. Many organics (e.g., solvents, pesticides, oils) may be incinerated or landfilled effectively. Some metals require conversion to compounds that will not react with other substances before being transferred to a landfill. Treatment options also can be troublesome as landfill space decreases and public opposition to incineration increases in some areas. However, effective air pollution controls are available to manage incinerator emissions, and engineering for landfill construction now includes sophisticated liners, leachate controls, and management practices to prevent groundwater contamination or other forms of cross-pollution.
Beyond excavation, more selective removal technologies have been developed for contaminants in soil, including soil washing, which uses processing equipment and chemical solvents to "wash" contaminants from soil. In practice, soil washing often is complicated and expensive. Phytoextraction—the use of plants to remove soil contaminants—has achieved favor in some applications. Selected plant species may remove and concentrate inorganic contaminants such as heavy metals and radionuclides in the above- or below-ground tissues. If phytoextraction is successful, the resulting plant tissue will have high levels of the soil contaminant and be classified as hazardous waste, requiring appropriate treatment or disposal options (see previous section). To date, phytoextraction has been used only at relatively small sites.
One of the best-documented cases of heavy metal phytoremediation in the United States was conducted at a former battery manufacturing site in Trenton, New Jersey. The land surrounding this urban facility that was in operation from the 1930s until 1980 was highly contaminated with lead. For two vegetation seasons Indian mustard plants were used to reduce the concentration of lead in the soil to below regulatory limits. This cleanup effort illustrates the potential for innovative, biological remediation technologies.
Sediments are the inorganic (e.g., clay, silt, sand) and organic (plant and animal) materials that settle to the bottom of water bodies. Aquatic sediments often become contaminated by a wide variety of man-made chemicals including agricultural chemicals such as pesticides that are washed into water bodies, industrial chemicals that are released into water bodies or that leak from containment structures as well as the many products that are transported by water. Contamination in aquatic sediments may affect the organisms that live within the sediments, or may bioaccumulate through the food chain as larger species feed on organisms that have absorbed the contamination. Remediating such contamination requires choosing between the risks associated with leaving the contamination in place and the risks associated with excavating the sediments (and resuspending them in the water), transporting and disposing of them.
Groundwater. Liquid or solid chemicals, when disposed of by burial or direct release onto the ground surface, can migrate down into the soil structure and come in contact with groundwater. Final disposition of these chemicals depends on their volatility and water solubility. Aqueous phase chemicals, chemicals that are soluble in water, dissolve in and move with groundwater. Nonaqueous phase chemicals (NAPLs) do not dissolve in water and may be either lighter than water (light nonaqueous phase liquids or LNAPLs) or heavier than water (dense nonaqueous phase liquids or DNAPLs). The distinction between DNAPLs and LNAPLs has a significant impact on the detection and remediation of organic contamination.
LNAPLs such as petroleum products (e.g., gasoline, diesel, oils) are common contaminants in urban, industrial, and agricultural areas. DNAPLs such as chlorinated solvents—trichloroethylene (TCE) and perchloroethylene (PCE)—are found also in urban and industrial areas, most commonly in association with the dry cleaning industry, where previous management practices often resulted in the spilling or dumping of these chemicals. These NAPLs pool above (LNAPL) or below (DNAPL) groundwater bodies, dissolving slowly into, and potentially contaminating, enormous volumes of water. In states that rely heavily on groundwater for drinking water, billions of dollars have been spent in the last two decades to replace leaking underground gasoline storage tanks (LUSTs) and to clean up historical contamination.
When contamination is detected in groundwater, one common cleanup approach is to drill wells, then pump out and purify the contaminated water using a variety of methods, including air stripping , where compounds are volatized from the water into the air. This technique does not rid the environment of the pollutants, however, as the contaminants are merely transferred from the water to the air. Less volatile compounds, or those at low concentrations, may be removed by filtration through a solid sorbent , such as activated carbon. This "pump and treat" approach addresses only the dissolved, aqueous phase of contamination, while leaving the concentrated, nonaqueous "pool" as a continuing source of groundwater contamination. As a result, "pump and treat" may be a prolonged process. The detection and elimination of NAPL source zones of contamination are more desirable where feasible.
In order to remove sources of groundwater contamination, technologies are needed to accurately detect and measure the amounts of these chemicals. Well drilling is commonly used to investigate or remediate contaminated sites, though it is relatively slow and expensive, and it brings up contaminated soil that must be disposed of properly. Direct push technologies use large vehicles equipped with hydraulic rams or percussion equipment to push metal tubes into the ground. Special sensors on the advancing tip of these tubes provide information on the nature of the sediments being penetrated. Recent advances in this technology allow special chemical sensors to be deployed on the end of the tube providing information on the presence and concentration of chemicals in the ground. The hollow tube also can be used to collect soil and groundwater samples. When sampling is complete, the rods typically are removed from the ground and the hole is sealed. While depth and geology limit "direct push," it is generally faster than well drilling, and it does not contaminate the soil.
Once source zones have been identified, technologies may be deployed to remove contamination. One of the most popular approaches to removing NAPLs is thermal treatment. Heating contaminated soil and groundwater to the boiling point of the contaminant will convert liquids to gases, which move through the soil. Wells are used to extract the resulting gases that can then be absorbed by activated carbon, or heated to temperatures high enough to break them down into harmless elements. Typically, soils are heated in one of two ways: electrical resistance or steam injection. Electrical resistance heating uses electrodes placed in the ground between which electrical currents are passed. The soil's resistance to the movement of the electrical current produces heat. Steam heating pumps high-pressure steam into the ground through injection wells.
Conversion
Conversion uses chemical reactions to change contaminants into less toxic or less mobile forms. These chemical reactions may be produced by the introduction of reactive chemicals to the contaminated area, or by the action of living organisms such as bacteria.
The use of biological systems to clean up contamination is known as bioremediation . Bioremediation includes all cleanup technologies that take advantage of biological processes to remove contaminants from soil and groundwater; the most common technique is microbial metabolism. For decades, scientists have known that microbes can degrade certain organic contaminants, and in cases of historical contamination, microbial communities often adapt to take advantage of the energy released when these chemicals are degraded (i.e., metabolized). By studying the existing conditions, substances that microbes need to break down chemicals, such as nutrients or oxygen, may be added to enhance biodegradation. Microbial biodegradation is capable of degrading most organic contaminants.
For example, under ideal conditions, microbes can degrade the organic constituents of petroleum hydrocarbons such as gasoline or diesel fuel, to carbon dioxide and water. This is the concept behind a technology being used by the U.S. Department of Energy to remove petroleum contamination from soils that also contain low levels of radioactive materials. The combination of hazardous materials (petroleum) and radiation places this soil in the regulatory category of mixed waste, for which disposal is extremely difficult. By using biodegradation to remove the petroleum component, the remaining soil can be classified as low-level radioactive waste, which has an accepted disposal mechanism.
Soils. Heavy metals are a common target for conversion approaches. Removal may not be practical when such metals contaminate large areas of surface soil. In these cases, chemical approaches often are sought to convert the metals to a less toxic and less mobile form. Such conversions often involve the use of reactive agents such as sulfur to create immobile sulfide salts of metals (e.g., mercury). Reducing the mobility of soil contaminants often refers to reducing the water solubility of the compounds. Reducing water solubility lowers the potential for contaminants to become dissolved in and move with water in the subsurface.
Groundwater. DNAPLs such as chlorinated solvents may be treated with chemicals (e.g., potassium permanganate) that degrade the solvents into relatively harmless chemicals. When combined with chlorinated solvents, potassium permanganate removes chloride ions , which results in the degradation of these chemicals to carbon dioxide (CO 2 ) and water. This technology holds promise as a tool for remediating these challenging contaminants.
Containment
Situations exist in which technologies are not available or practical to remove or convert contaminants. In those situations, it is often possible to contain the contamination as a final solution or as an interim measure until appropriate technologies become available.
Soils. Radionuclides from historical weapons production and nuclear testing, as well as from industrial uses of radiation, appear to be a good match for developing containment technologies. For example, containment is a promising technology for the management of radioactively contaminated soils beneath the large high-level radioactive waste storage tanks at the U.S. Department of Energy Hanford site in Washington State. Removing radioactive contamination from soil is problematic from a worker-safety standpoint, and it may create further contamination of equipment, containers, and surrounding areas. Efforts to develop effective physical containment technologies for soil contaminants are continuous.
Groundwater. Groundwater is not generally suitable for absolute containment; however, between containment and conversion is a technology known as reactive barriers. Reactive barriers intercept contaminated groundwater plumes and are constructed of chemically reactive materials (e.g., iron) that bind or convert dissolved contaminants. Reactions between the contaminant and the iron either immobilize or degrade the contaminant by altering its chemical form (redox manipulation).
Passive Cleanup
Passive remediation technologies are increasingly common in some applications, and take advantage of naturally occurring chemical or biological processes that degrade contaminants to less toxic forms. The accepted term for this group of technologies is monitored natural attenuation (MNA), which is the result of regulatory recognition that natural biological processes are capable of degrading certain contaminants under specific conditions and that dispersion may aid in achieving objectives. MNA is employed for the cleanup of organic contaminants such as petroleum hydrocarbons in situations where the longer time frame associated with MNA does not increase the risks posed by the contamination. MNA recognizes that, while these processes are possible, they must be monitored to insure that the expected progression actually occurs. In the State of Florida, MNA is being used as an approved cleanup action for some former dry cleaning sites. At these sites, natural processes are being monitored as they degrade chlorinated solvents from the former dry cleaning operations.
MNA is one example of major innovation in this area. Environmental cleanup is a dynamic field. Advances in science and engineering fuel innovative approaches and technologies, and advanced technologies provide greater capabilities in meeting the ultimate goal of a safer and healthier environment.

